The world has seen multiple pandemics of influenza over the centuries. The 20th century saw three, while there was one in the early years of the 21st century, the H1N1 pandemic influenza of 2009. While there are several types and subtypes of the influenza virus, only type A has produced pandemics in the human population. Although the timing of the next influenza pandemic cannot be predicted, preparations can be put in place to respond efficiently to one when it is declared to minimize the impact. As such, guidance has been developed by the World Health Organization for its members in their implementation of preparedness plans. Canada’s most recent influenza preparedness plan was published in 2018. While the Public Health Agency of Canada plays a central role in Canada’s pandemic influenza plan, preparedness involves collaboration at all levels of government of this country as well as internationally. Lessons learned from Canada’s response to the non-influenza COVID-19 pandemic and subsequent changes made to the Public Health Agency of Canada’s pandemic preparedness infrastructure will have an impact on how Canada responds to the next influenza pandemic.
On 11 June 2009, the World Health Organization (WHO) declared the first global influenza (flu) pandemic since 1968. A pandemic is an outbreak of a specific illness in multiple areas covering a vast geographic area. While the world experienced a pandemic that began in 2020 due to a coronavirus (SARS-CoV-2), this paper focusses on pandemic influenza. It describes flu viruses and the flu pandemics that have afflicted humankind and discusses the influenza preparedness plans of the WHO as well as Canada.
The flu is caused by viruses that infect the respiratory tract of mammals and birds. Compared with most other viral respiratory infections, such as the common cold, influenza infection often causes a more severe illness. Typical symptoms of the flu include fever, cough, sore throat, runny or stuffy nose, headache, muscle aches and often extreme fatigue.1 Stomach flu is not influenza.2
Viruses are unique organisms that cannot be clearly categorized as either living or non-living but are generally considered to be non-living.3 They contain genetic material but can reproduce only by infecting another organism, that is, by attaching to and injecting their own genetic material into a host’s cell. The host’s genetic replication machinery is then “hijacked” to produce multiple copies of the various viral components, which are repackaged as intact viruses that then leave the cell and go on to infect more host cells.4
The flu virus contains RNA (ribonucleic acid) as its genetic material (rather than deoxyribonucleic acid, or DNA, found in all other forms of life) and can be divided into types A, B, C and D based on protein differences. Only types A and B cause illness in humans, including significant illness and death. Only type A influenza viruses have been associated with pandemics.5
Type A flu viruses are known to include several subtypes. These subtypes relate to differences in proteins on the virus’s external surface. These surface proteins are the principal target of the immune response. The subtype names relate to the two surface proteins that vary from one subtype to the next: “H” refers to the protein hemagglutinin, and “N” refers to the protein neuraminidase. No subtypes of type B influenza virus are known.
When exposed to a flu virus, our immune system responds by producing antibodies to the surface proteins. An effective response results in the elimination of the virus from our bodies. In order to survive, viruses must avoid being destroyed by antibodies, which is accomplished by two mutation mechanisms that bring about changes to the surface proteins. One type of mutation, called antigenic drift, introduces very small changes into the surface proteins; many of these changes do not affect the immune response. This type of mutation is sufficiently slow that seasonal flu vaccines can provide some degree of protection. However, the second type of mutation, called antigenic shift, introduces large changes in the viral proteins and may also occur when a virus infects a different species. These large mutations happen only in type A flu viruses and pose the greatest risk for human flu pandemics.6
There have been several flu pandemics over the centuries. As population density has increased and global travel become more frequent, viral infections have spread further and faster. There were three flu pandemics in the 20th century and, most recently, one in 2009. These pandemics are summarized in Table 1.
In 2009, the most recent flu pandemic was declared when a new strain of subtype H1N1 of swine origin was detected in two children in California. The unique genetic sequence of the new strain permitted easy transmission of the virus among people and caused significant illness. H1N1 spread quickly around the world. By the end of June 2009, over 77,000 cases had been confirmed in 116 countries – within two weeks of the WHO’s announcing a global influenza pandemic, the first pandemic in the 21st century. Canada confirmed 7,983 cases of H1N1 influenza resulting in 538 hospitalizations and 25 deaths.7
| Pandemic/Epidemic | Responsible Virus | Infections/Fatalities | Most Affected Population |
|---|---|---|---|
| Influenza pandemic of 1918–1920 | Influenza type A virus, subtype H1N1 | Estimated 500 million infected, 50 100 million deaths | Children and young adults |
| Influenza pandemic of 1957–1958 | Influenza type A virus, subtype H2N2 | 1.1 million deaths | Children |
| Influenza pandemic of 1968–1969 | Influenza type A virus, subtype H3N2 | 1 million deaths | Children and young adults |
| Influenza pandemic of 2009–2010 | Influenza type A virus, subtype H1N1 (A(H1N1)pdm09) | 200 million infected, 200,000 deaths | Adolescents and young adults |
Sources: Table prepared by the Library of Parliament using data obtained from N. J. Cox and K. Subbarao, “Global Epidemiology of Influenza: Past and Present,” Annual Review of Medicine, Vol. 51, 2000, pp. 412–413; and “Table 1 – A summary of transmission of CoVs and IAVs” in Chao Jiang et al., “Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season,” Microbes and Infection, Vol. 22, 2020, p. 239.
The WHO Global Influenza Surveillance and Response System (GISRS) is used to help the WHO recommend the content of the influenza vaccine for the upcoming influenza season. It also serves as a global alert mechanism for the emergence of influenza viruses with pandemic potential. The network, first created in 1952, has established a system of laboratories that enable the WHO to provide the following:
The components of the WHO GISRS are
Surveillance information is uploaded to the WHO’s surveillance database, FluNet, by the participating centres and laboratories listed above. FluNet is used to track the global spread of influenza viruses.9 GISRS and FluNet track both seasonal and pandemic influenza.
In 2019, the WHO released its Global Influenza Strategy aiming to improve global control of seasonal influenza, reduce the spread of influenza viruses from animals to humans and improve pandemic influenza preparedness.10 The WHO issued its first guidance specific to pandemic influenza in 1999,11 and there have been a number of updates and revisions to the guidance since that time. The most recent WHO guidance, entitled Pandemic influenza risk management: A WHO guide to inform and harmonize national and international pandemic preparedness and response, was issued in 2017.12 The guide was previously revised in 2013 to take into account lessons learned from the 2009 H1N1 influenza pandemic. It uses a whole-of-society approach that acknowledges the roles of various stakeholders – national governments, health sectors, non-health sectors and individuals – in mitigating the effects of a possible pandemic. The guide describes the role of the International Health Regulations, identifies overlapping pandemic phases and discusses vaccine production. As well, it is intended to be used in conjunction with the Pandemic Influenza Preparedness (PIP) Framework.
The PIP Framework, overseen by the World Health Assembly, was first issued in 2011 with a second edition issued in 2021.13 It is a “public health instrument that brings together member states, industry, other stakeholders and WHO to implement a global approach to pandemic influenza preparedness and response.”14 It involves collaboration among member states to share biological material and information within GISRS about viruses with pandemic potential and, under the Partnership Contribution mechanism, contributions from manufacturers of vaccines, medicine and diagnostics for improving pandemic influenza preparedness and more equitable access for member states in need of pandemic vaccines and medicines used in a pandemic.15
The pandemic phases identified by the WHO as guidance to member states are summarized in Figure 1.
Figure 1 – Pandemic Influenza Phases described by the World Health Organization
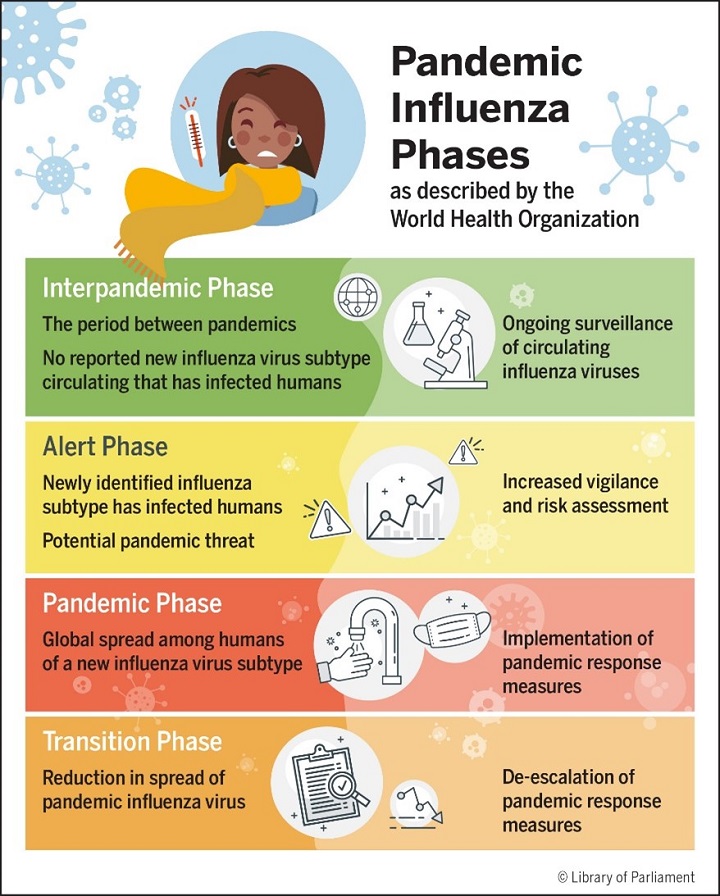
Source: Figure prepared by the Library of Parliament using information obtained from World Health Organization, Global Influenza Programme, Pandemic influenza risk management: A WHO guide to inform and harmonize national and international pandemic preparedness and response, 2017, pp. 13–14.
The Canadian Pandemic Influenza Plan for the Health Sector was issued in 2004 and then updated by the Pan-Canadian Public Health Network in 2006 and intended as an evergreen document. The plan was put into action during the 2009 H1N1 influenza pandemic. An internal review of the response to that pandemic suggested that it was successful overall and that planning effectively reduced the impact of the virus. However, the report recommended improving surge capacity, as Canada’s infrastructure could have been overwhelmed if the H1N1 virus had been more infectious or if it had produced illness in a broader population base. In addition, it recommended improving science-based communications from Health Canada and the Public Health Agency of Canada (PHAC).16
In 2018, the document was replaced with Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector (CPIP), with lessons learned from the 2009 flu pandemic included in the new version.17
The CPIP is based on basic principles of public health and emergency response and follows a whole-of-society approach. Its goals are to minimize illness and death while also minimizing social disruption, efforts that require collaboration and coordination of activities by all levels of government.
The CPIP employs the WHO phases described above only as guidance for identifying triggers for action. The CPIP states:
Canada’s response to the novel/pandemic virus will relate to its presence and activity levels in this country, which may not coincide with the global picture. Therefore, the WHO global phases will not be used to describe the situation in Canada or be used as triggers for action in Canadian jurisdictions.18
The triggers for action described in the CPIP are summarized in Table 2.
| Trigger | Action | Comment |
|---|---|---|
| A novel influenza (flu) virus identified elsewhere in the world but little or no spread |
|
Tailor communications to the health sector and the public; to be continued throughout the pandemic response |
| Sustained transmission of a novel flu virus elsewhere in the world |
|
Flu pandemic may be imminent or already underway |
| A novel flu virus with sustained transmission elsewhere first detected in Canada |
|
Emergency response activities may have been activated at previous trigger, depending on circumstances |
| Spread of novel pandemic flu virus within provincial/territorial or local jurisdiction |
|
Level of activities will increase or decrease depending on infection rates |
| Spread of flu infection stretches capacity to provide services |
|
This trigger may not be reached by all jurisdictions |
| Flu infection rate slows, easing demand on services |
|
Not applicable |
| Vaccine against novel flu virus becomes available |
|
Not applicable |
| Second and subsequent waves of flu infection |
|
Not applicable |
| End of flu pandemic |
|
Incorporate lessons learned from the pandemic into future revisions of pandemic plans |
Source: Table prepared by the Library of Parliament using information obtained from Pan-Canadian Public Health Network, “Table 2 – Pandemic Triggers and Typical Accompanying Action,” Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector ![]() (2.60 MB, 64 pages), 2018, p. 38.
(2.60 MB, 64 pages), 2018, p. 38.
The CPIP also discusses the components of pandemic preparedness and response referred to in Table 2. Table 3 summarizes those components, additional details for which are provided in separate annexes to the CPIP.19
| Component | Purpose | Comment |
|---|---|---|
| Surveillance | To monitor geographic spread of cases, infection rates and trends, impact (cases, hospitalizations, deaths, health system demands, changes to the influenza (flu) virus in question) | Provides the information needed to make timely decisions about response strategies |
| Laboratory services | To identify first flu cases, provide data on infections and viral characteristics needed for surveillance activities | Needed for identification of novel flu virus, development and implementation of diagnostic testing to track virus activity throughout the pandemic |
| Public health measures | To reduce the rate of flu infections and the burden on the health care system until a vaccine is available | Non-pharmaceutical interventions including masks, social interactions and travel restrictions |
| Vaccines | To prevent illness in individuals and ultimately the spread of flu infections to end the pandemic | Canada’s pandemic vaccine strategy aims to provide, distribute, administer authorized vaccines and to monitor them for safety and effectiveness |
| Anti-viral medications | To treat infected individuals and prevent illness in persons exposed to the flu virus | The only flu specific drug treatment available; can be used before vaccines are available |
| Infection prevention and control, occupational health | To prevent exposure to and transmission of pandemic flu during the provision of health care | Requires the collaboration of officials responsible for providing occupational health programs and infection prevention and control programs |
| Health care services | To maintain acceptable levels of service for all patients during surges of flu cases | Requires innovative approaches including triage, virtual and phone based care, online resources |
| Clinical care guidelines | To ensure all practitioners are provided with necessary and up-to-date guidance on the care of patients with pandemic flu | Patients may have a range of symptoms |
| Communications | To provide information that is consistent and easy to understand about the virus, the illness and spread of the disease | Open and transparent communication is critical to optimize the public’s compliance with public health measures |
| Research | To develop effective treatments and vaccines and to track the evolution of the virus | Research should also be ongoing during interpandemic periods |
Source: Table prepared by the Library of Parliament using information obtained from Pan-Canadian Public Health Network, “4.0 – Key Components of Pandemic Influenza Preparedness and Response,” Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector ![]() (2.60 MB, 64 pages), 2018, pp. 41–53.
(2.60 MB, 64 pages), 2018, pp. 41–53.
Under the Constitution, health and health care are shared responsibilities between the federal, provincial and territorial governments.20 As such, all levels of government have a role to play during public health emergencies, including during pandemics. The federal government plays a critical role in collaborating with provincial and territorial governments as well as international efforts to optimize the effectiveness of the pandemic response. In addition, it is responsible for ensuring that all federal departments have implemented emergency plans and that international borders are protected to minimize the introduction of infectious disease into the country.21
The federal government’s role in the CPIP is carried out primarily by PHAC. PHAC’s Centre for Emergency Preparedness and Response (CEPR) and its Health Portfolio Operations Centre coordinate with federal departments, other levels of government and other stakeholders under the Federal/Provincial/Territorial Public Health Response Plan for Biological Events to provide emergency management services.22
National surveillance of influenza and influenza-like illness (ILI) is carried out by PHAC under its FluWatch program, which collects information through a network of labs, hospitals, doctors’ offices and provincial and territorial ministries/departments of health. FluWatch reports are produced weekly throughout the year. The FluWatch program lists seven components of Canada’s influenza surveillance:
PHAC’s CEPR maintains the Global Public Health Intelligence Network (GPHIN), which is a secure, Internet-based early warning system that intends to gather preliminary reports of public health significance by monitoring global media sources on a real-time, 24/7 basis. Notifications about events that may have serious public health consequences are forwarded to registered users such as government authorities, non-governmental public health agencies and other stakeholders. This system is not limited to influenza and can include other infectious diseases, incidents of contaminated food and water, bioterrorism and exposure to chemical and radio-nuclear agents, and natural disasters.24
The effectiveness of GPHIN to identify the emerging COVID-19 threat in late 2019 and early 2020 has been the subject of criticism. Media reports in the summer of 2020 suggested that changes within PHAC resulted in reduced international surveillance and the failure of GPHIN to issue international alerts.25 A March 2021 report of the Auditor General of Canada on Canada’s pandemic preparedness noted GPHIN’s failure to issue an early warning about the COVID-19 virus.26
On 17 August 2020, the Minister of Health established an independent panel to review GPHIN. The review examined GPHIN’s contributions to domestic and international public health intelligence including its role in PHAC’s early response to COVID-19, its current effectiveness, and its future role in event-based public health surveillance. The final report of the review, issued in July 2021, made numerous recommendations regarding the mandate, vision, governance, partnerships, roles and responsibilities of GPHIN. It emphasized that the identification of early warning signals and the issuance of alerts should remain the core functions of GPHIN.27
PHAC’s CEPR also funds and maintains a national emergency strategic stockpile (NESS), which includes a central depot in Ottawa and additional warehouses located across Canada. The NESS provides emergency supplies, usually within 24 hours, to provinces and territories when requested. The NESS contains hospital supplies such as beds and blankets, hospital and personal protective equipment (PPE), medical devices, and pharmaceuticals such as antibiotics and antivirals, as well as mini clinics, which are collections of NESS items that can be deployed for triage and to reduce demands on local medical services and can be set up in existing buildings such as schools and community centres.28
Antiviral stocks are maintained in the NESS as well as in the National Antiviral Stockpile that was established in 2004 and is managed by the provinces and territories.29 Vaccines cannot be stockpiled because they can be prepared only once a circulating virus strain has emerged and been identified. Instead, Canada entered into a 10-year contract with GlaxoSmithKline in 201130 for sufficient vaccine for all Canadians in the event of a flu pandemic.31 In May 2021, the Auditor General of Canada issued a report on PHAC’s NESS. The report noted that there had been long standing problems that remained unaddressed at the outset of the COVID-19 pandemic. As a result, PHAC was not prepared to respond to the high demand for PPE and medical devices from the provinces and territories, although the report acknowledged that PHAC took action to improve “how it assessed needs and purchased, allocated, and distributed equipment.”32
In December 2021, PHAC released its 2021 annual report entitled A Vision to Transform Canada’s Public Health System. The report made a number of observations concerning changes needed to better prepare Canada’s public health infrastructure for future pandemics including improved collection and sharing of health data, greater collaboration between all levels of government and stakeholder groups in Canada, updated surveillance systems, an equity approach to pandemic response, and greater surge capacity in hospitals and other health resources.33
Pandemic influenza plans were updated in Canada and internationally following the 2009 H1N1 pandemic. Canada’s responses at the beginning of the non-influenza COVID-19 pandemic highlighted some deficiencies in preparedness plans for public health threats which the Government of Canada has started to address.
© Library of Parliament